Information
A major theme in graph-theoretic approaches is comparing nodes by different centrality measures. There are hundreds of centrality measures, some of which measure similar properties. Here are some selected measures we use that have biological relevance. By default interactive network objects will compute degree, eigen, betweenness, and stress centrality. Below are all available centrality measures in the package.
Source: https://www.centiserver.org
Jalili M, Salehzadeh-Yazdi A, Asgari Y, Arab SS, Yaghmaie M, Ghavamzadeh A, Alimoghaddam K. (2015) CentiServer: A Comprehensive Resource, Web-Based Application and R Package for Centrality Analysis. PLoS ONE 10(11): e0143111. DOI: 10.1371/journal.pone.0143111
Application of Centality Measures
yeast.networks <- readRDS(file.path(system.file("extdata", package="bieulergy"), "yeast-networks.rds"))
names(yeast.networks)[1] "Yeast_1" "Yeast_2" "Yeast_3"
# Working with single network
network <- yeast.networks$Yeast_1
# Working with multiple network
networks <- yeast.networksImportant Measures
One of the first things you might do for a network is check to see which centrality measures are responsible for the variance among nodes.
network.pca.pltvar(network)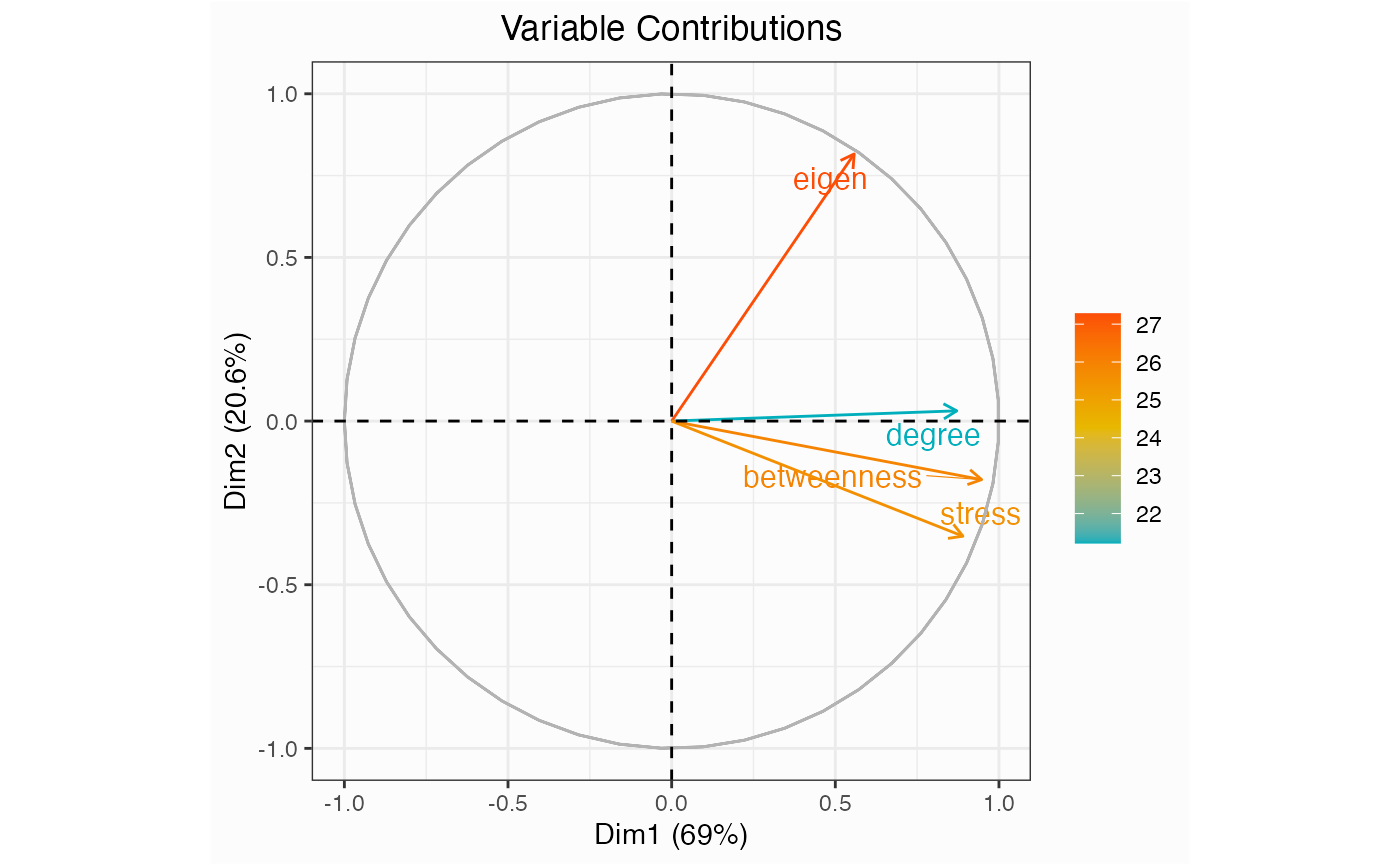
network.pca.contri(network)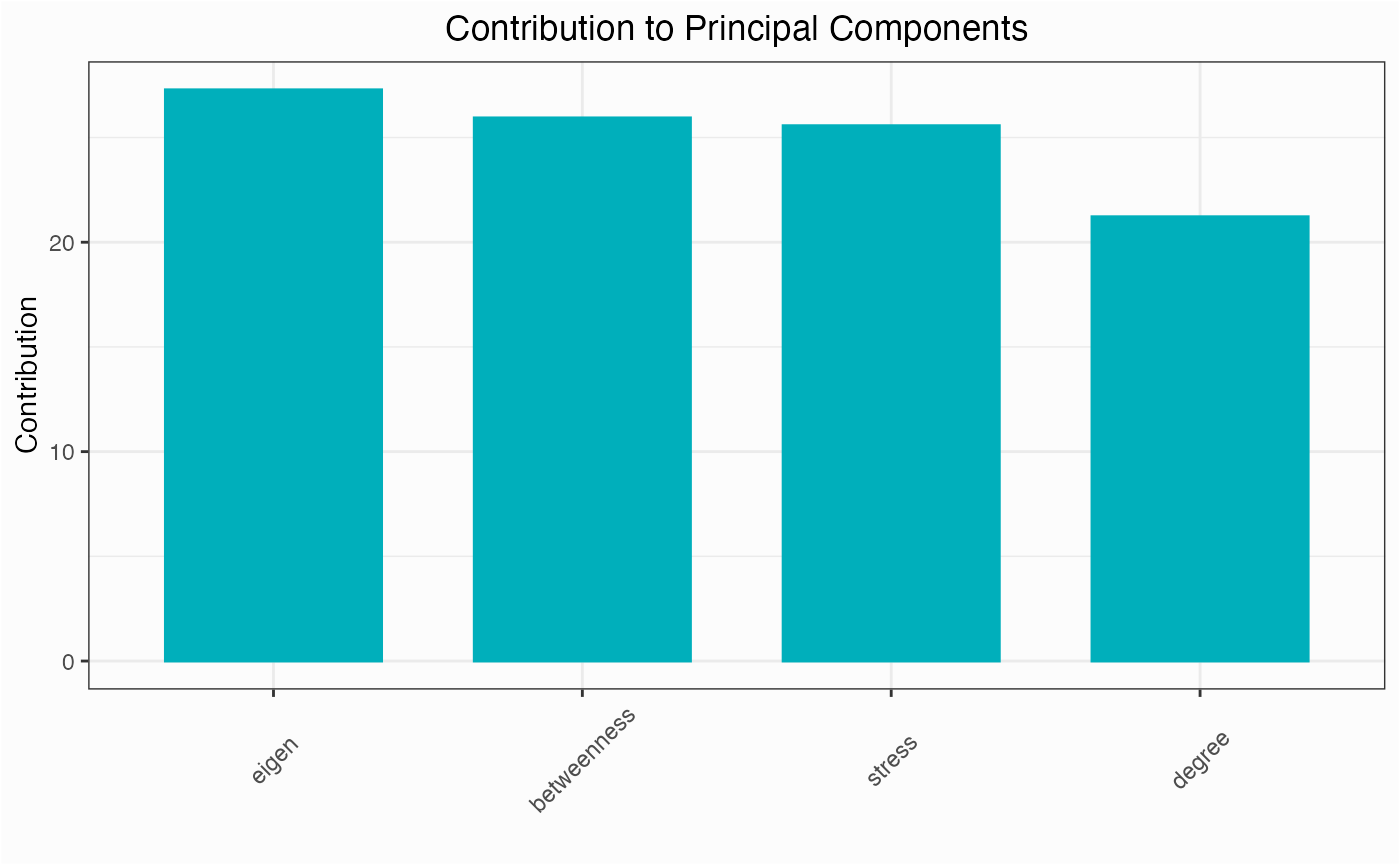
network.pca.varexp(network)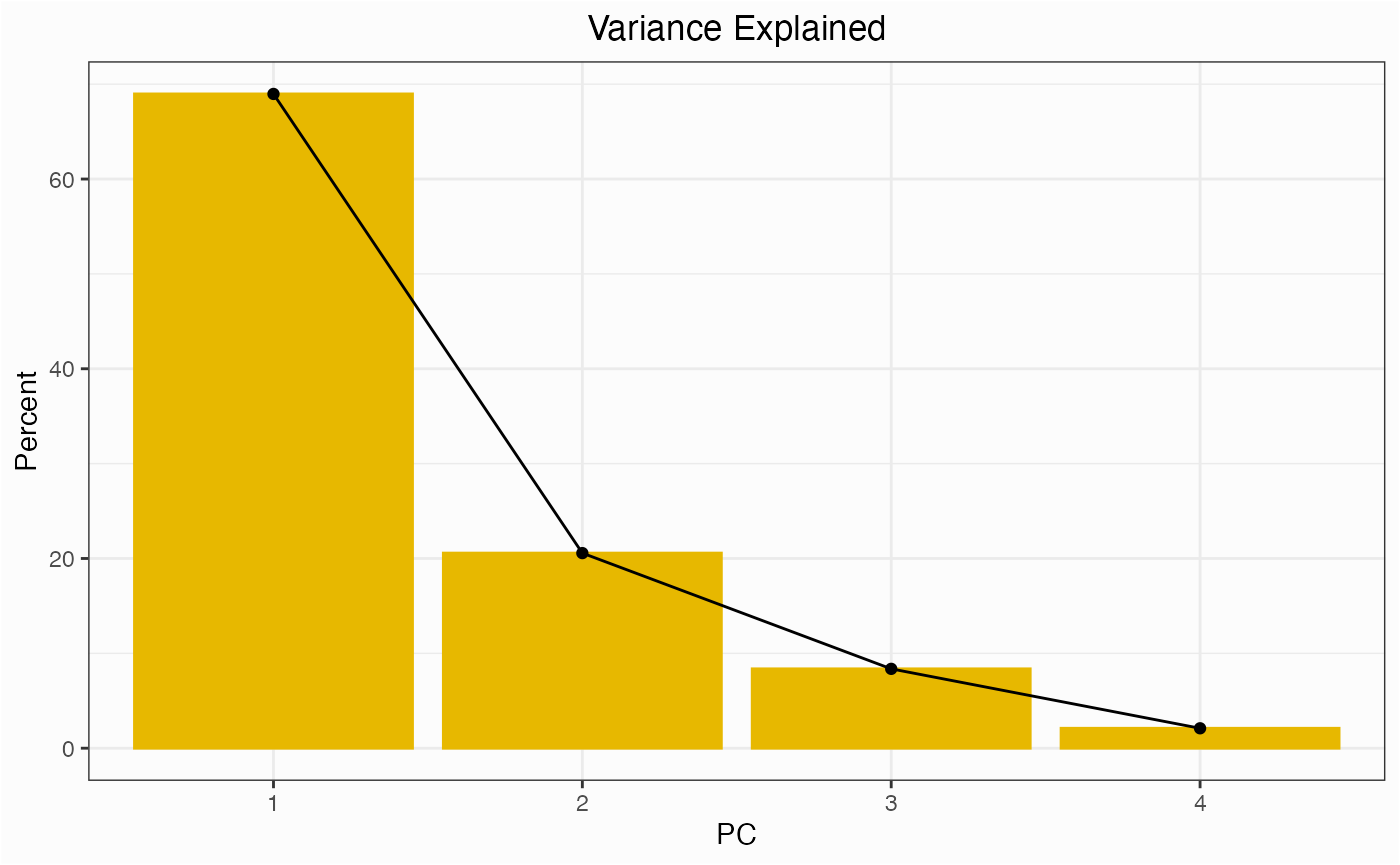
network.pca.hclust(network)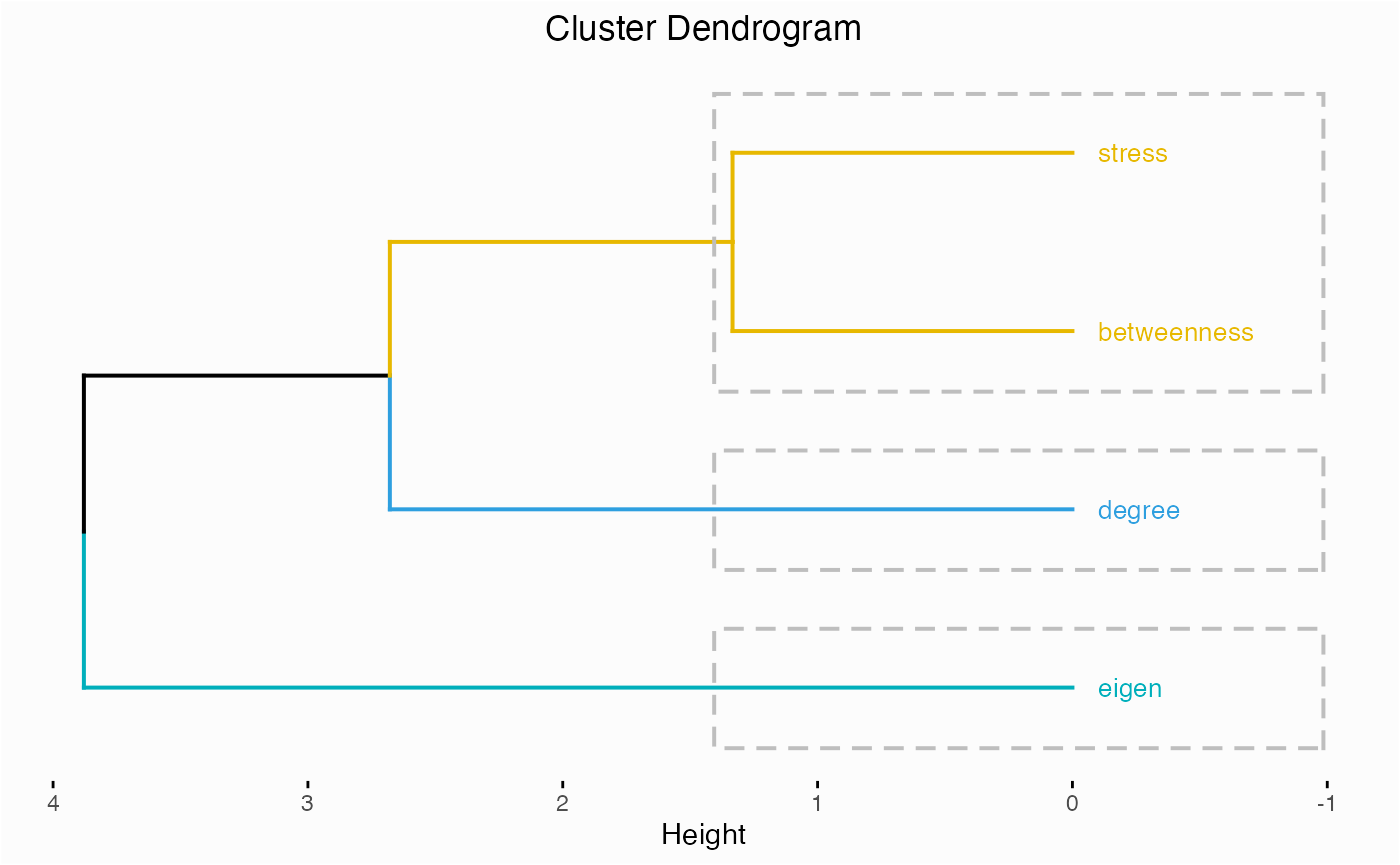
Centrality Distribution
networks.cdistr(networks, metric="degree")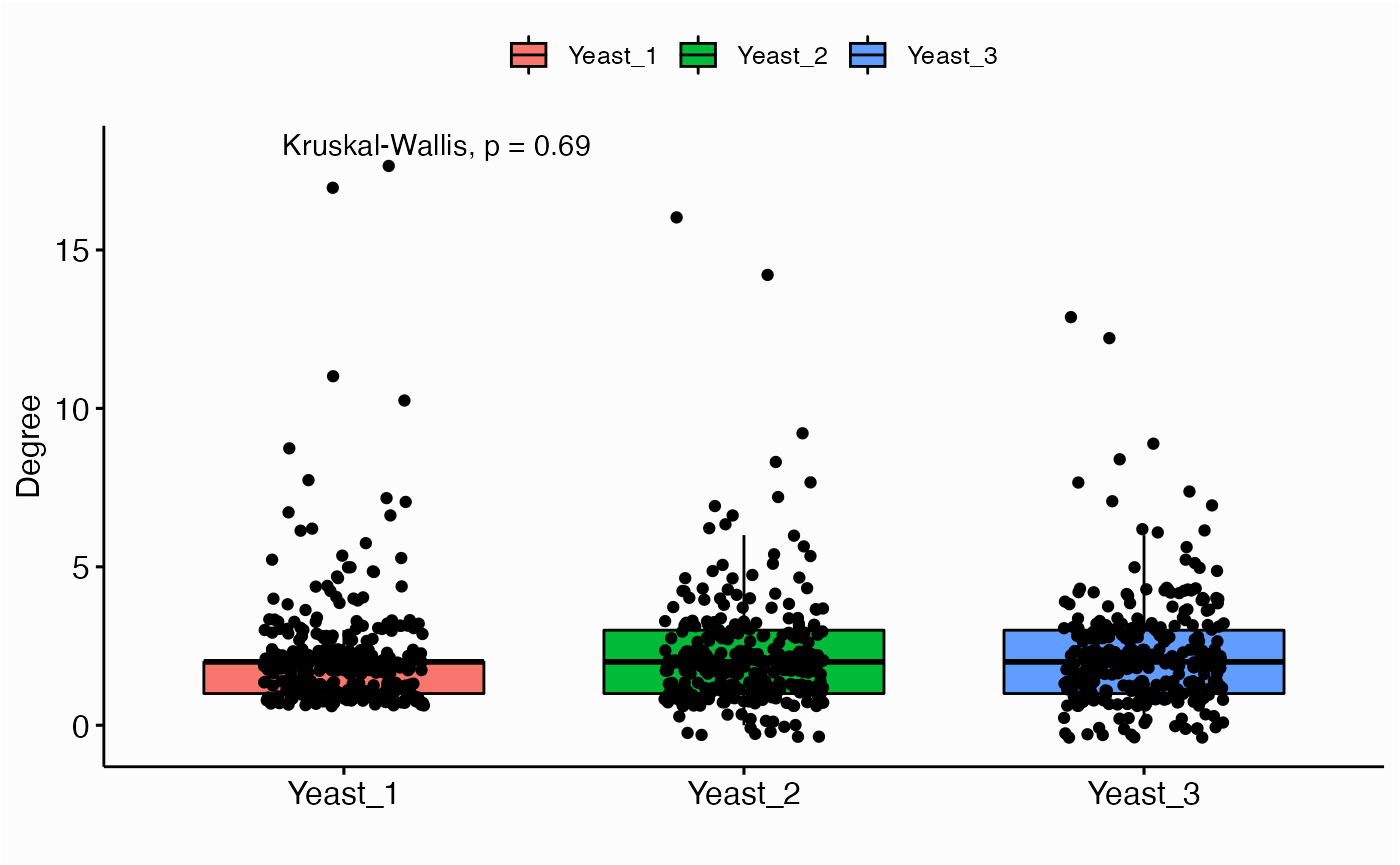
networks.cdistr(networks, metric="eigen", plot="density")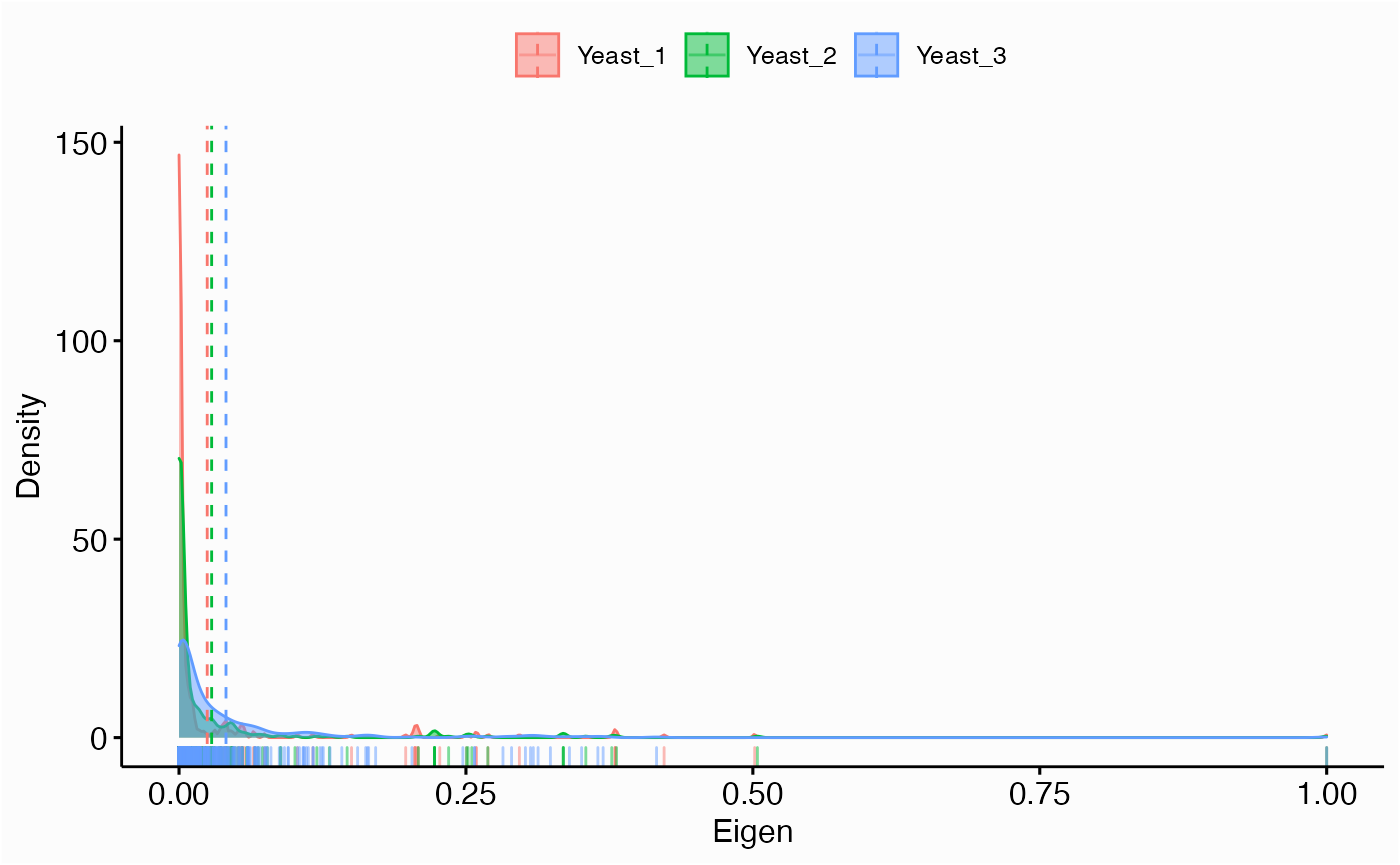
networks.cdistr(networks, metric="eigen", plot="ecdf")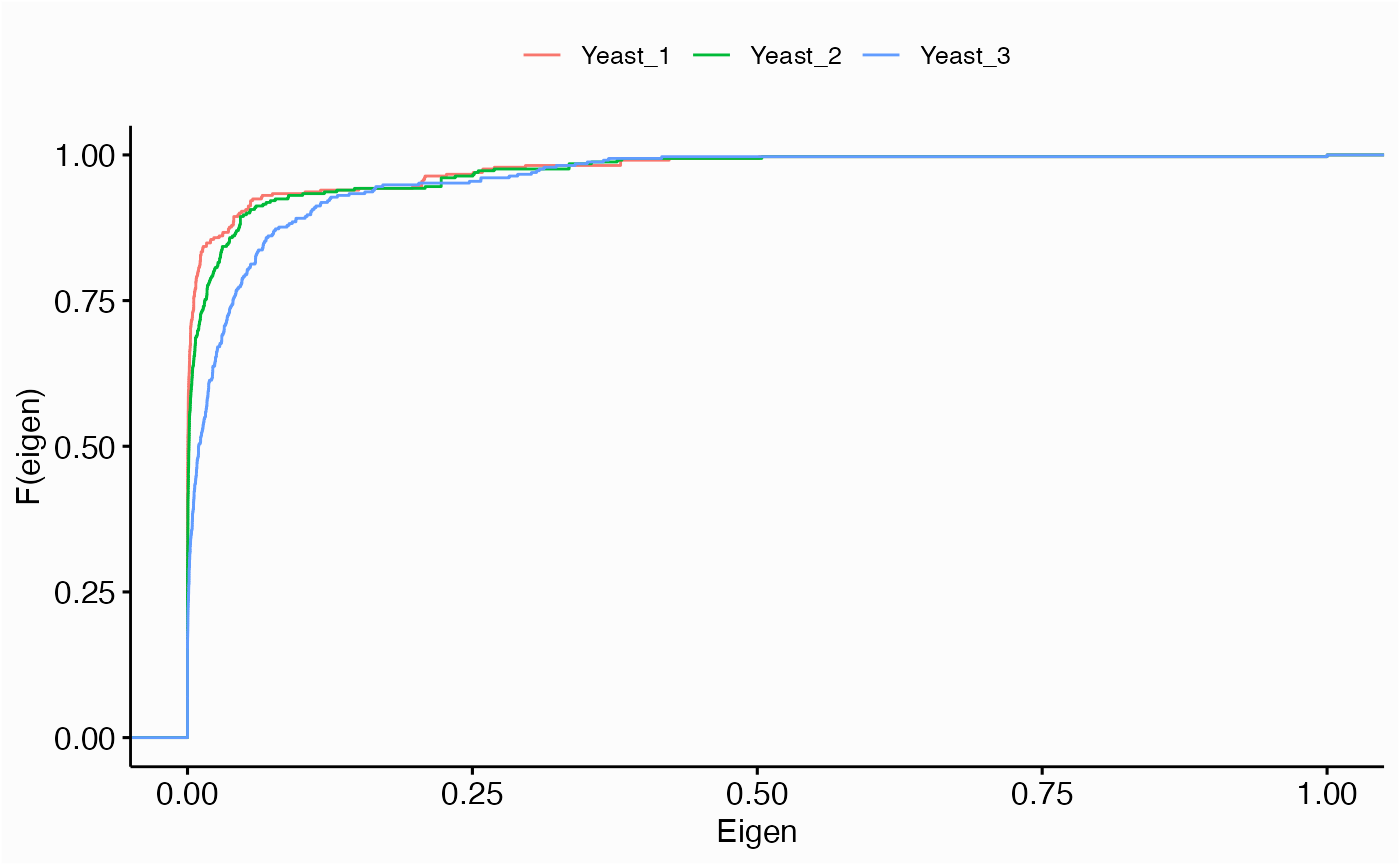
Centrality-based Enrichment
library(hypeR)
genesets <- enrichr_download(genesets="KEGG_2019", db="YeastEnrichr")
geneset <- genesets$`MAPK signaling pathway`
head(geneset)[1] "RGA1" "RGA2" "STE50" "MSS11" "MID2" "DIG1"
yeast.1 <- yeast.networks$Yeast_1
network.kstest(yeast.1, metric="eigen", geneset=geneset)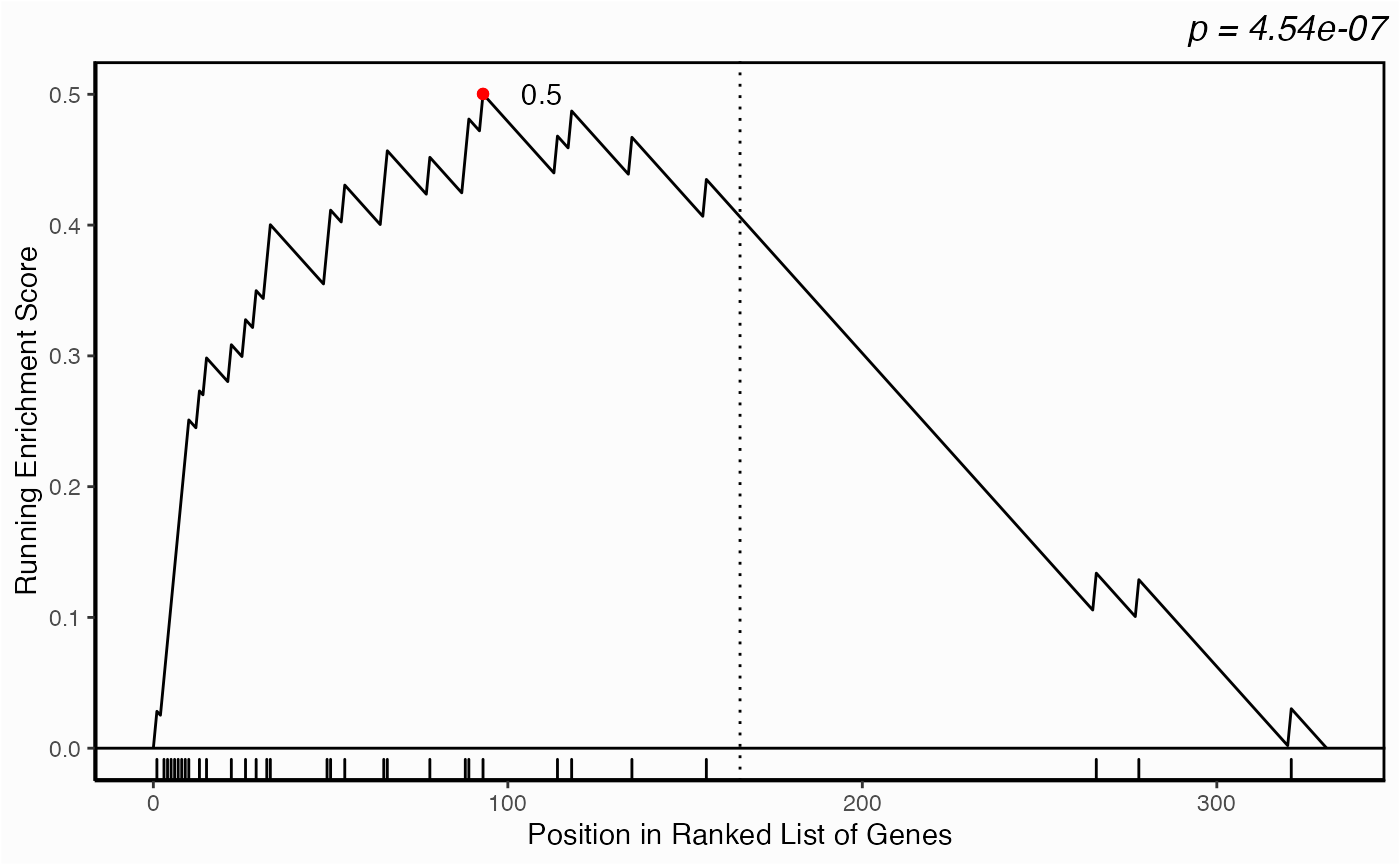
p <- lapply(yeast.networks, function(x) network.kstest(x, metric="eigen", geneset=geneset))
p <- mapply(function(px, label) {
px+ggplot2::labs(subtitle=label)
}, p, names(yeast.networks), SIMPLIFY=FALSE)
ggpubr::ggarrange(plotlist=p, ncol=1, nrow=3)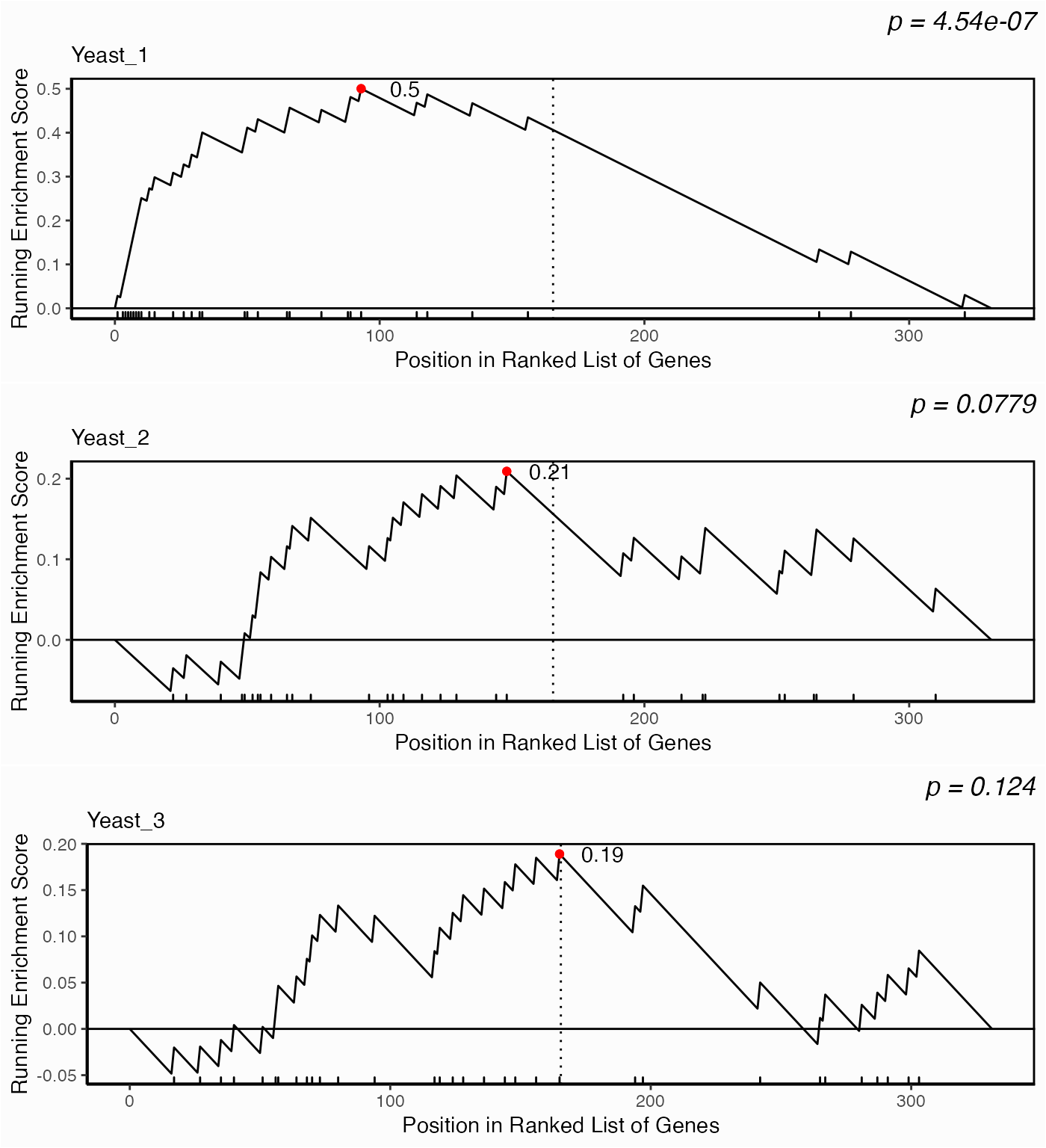
Comparing Centrality Ranks
ggwrs(100, p=5)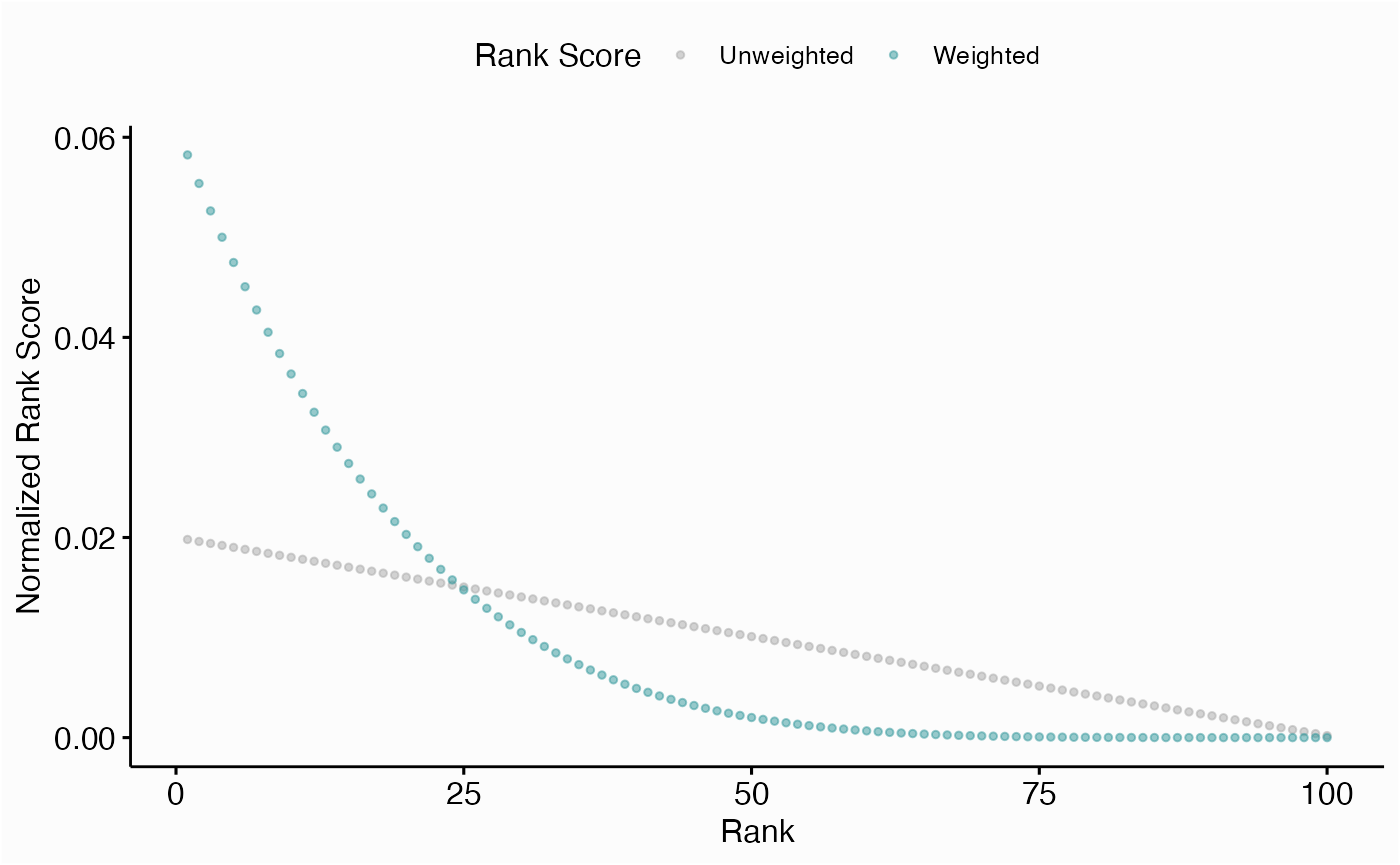
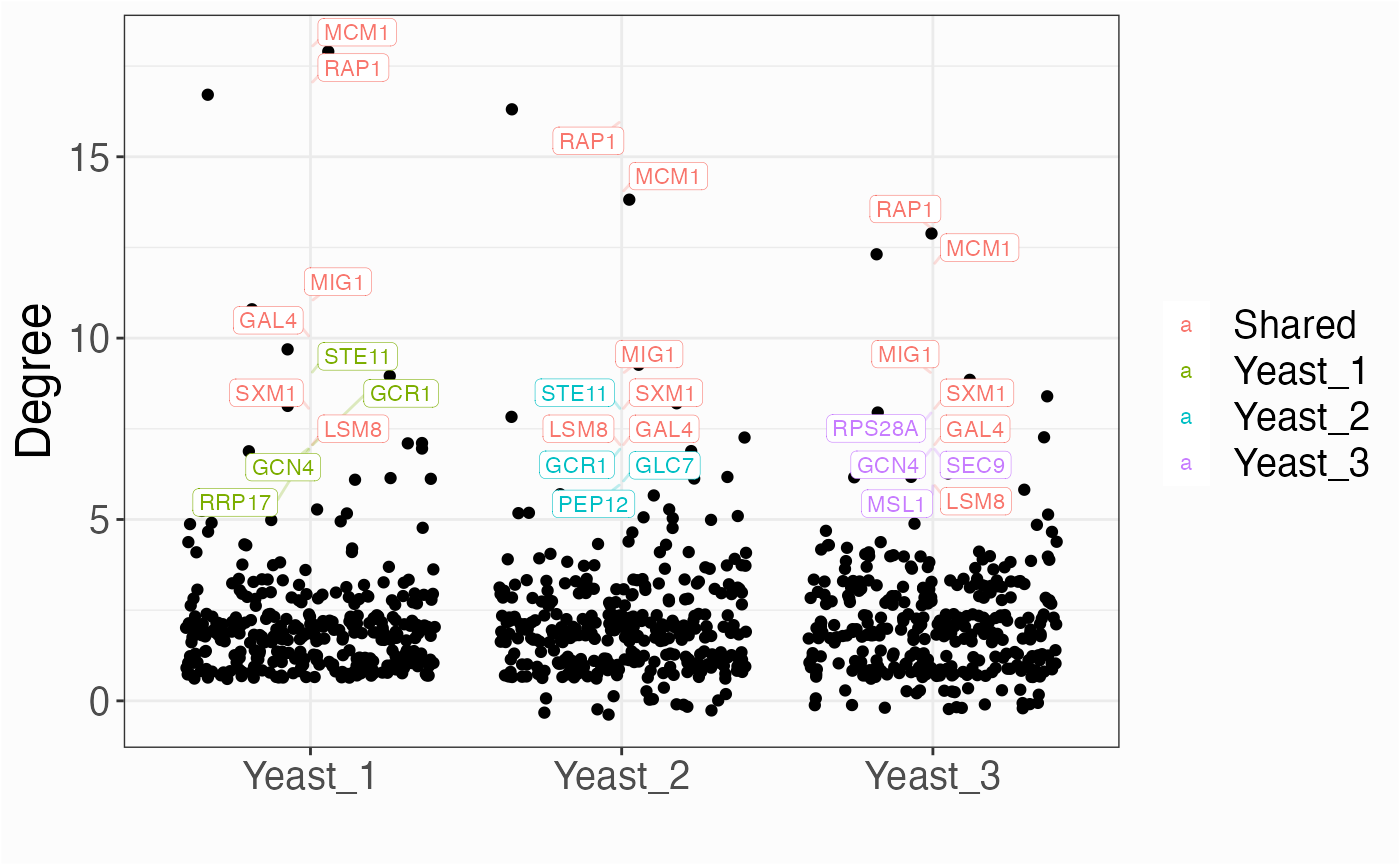
networks.diffc(yeast.networks, metric="eigen", top=25, p=5)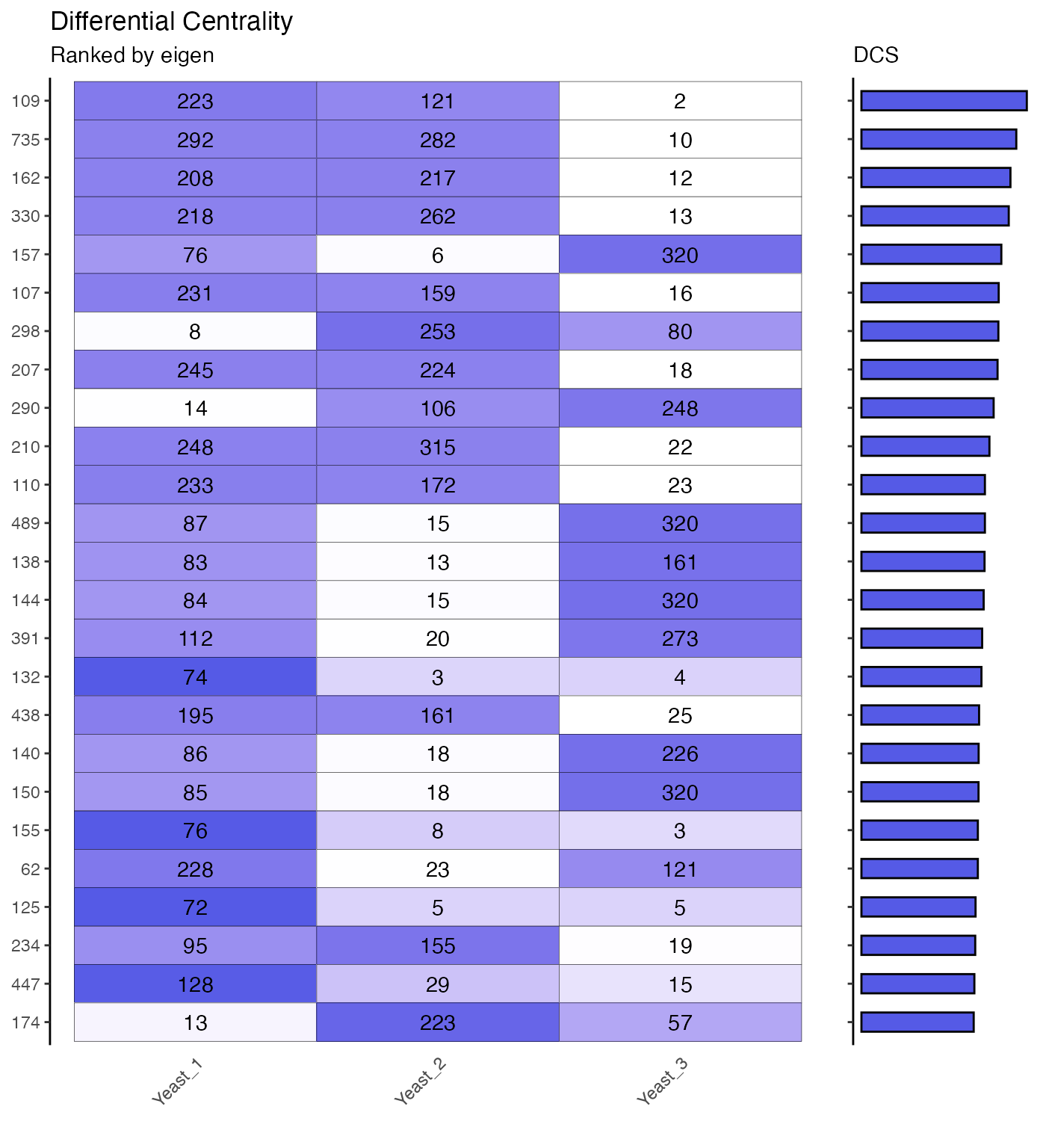
yeast.3 <- yeast.networks$Yeast_3
yeast.3$get.symbols("109")[1] "LSM8"